Bone Anchored Hearing Aid (BAHA)
This is our most common type of surgically implanted bone conduction hearing aid. It includes the implant (4mm titanium screw that is drilled into the skull), the abutment (protrudes through the skin), and the external sound processor (picks up and amplifies sound before converting it to mechanical vibrations that are sent through the abutment to the internal implant).
Cochlear (“BAHA”) and Oticon Medical (“PONTO”) both make versions of this type of device.

Benefits
Allows for a direct pathway for sound to travel from the outside environment to the cochlea/auditory nerve because the abutment protrudes through the skin, so there is no barrier to impede sound travel. These devices are also available in standard, power or super power amplifiers, allowing people with more severe degrees of hearing loss to wear successfully.
Risks
Chronic skin issues can develop in 5%-10% of users. Infection to implant site is common, so the site must be maintained with a rigorous hygiene regimen. Cosmetically unappealing to many younger users since there is a visible abutment when not wearing the device. Physical contact with hats, helmets, scarves and collars can cause excessive feedback (squealing and whistling).
Active Transcutaneous Device (OSIA)
This is an emerging technology that we have begun to adopt for the majority of cases. It includes the exact same 4mm titanium implant as the BAHA to conduct the sound through the skull to the cochlea. The implant is attached to a receiver-stimulatorthat generates sound. The external sound processorpicks up and amplifies the sound before transferring internally to the receiver-stimulator via electromagnetism.
To date, only Cochlear makes this type of specific type of device (Med-El makes something technically similar, but the implant is not osseointegrated). Oticon Medical will release a version by 2023.
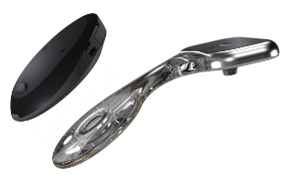
Benefits
Allows for a direct pathway for sound to travel from the outside environment to the cochlea/auditory nerve because the amplified signal is generated under the skin by the receiver-stimulator. Fewer chronic skin issues/infections associated with this device since there is no abutment or open wound. Cosmetically more appealing to patients. No feedback. Waterproof options available.
Risks
There is only one power/volume option, so it is unsuitable for those with more severe hearing losses as users may lack access to soft and moderate sounds necessary for speech understanding.


